New Wearable Ultrasound Treatment Can Reach Rural Populations With Joint Pain
Pain is among the top reasons that people go to a doctor.1 It affects quality of life and can lead to depression, anxiety, and poor sleep. Medications are often the first-line treatment for pain, but they can also cause serious side effects. Alternative treatments exist but are not available to all populations, especially rural and socioeconomically disadvantaged people.
“It's a significant challenge for rural patients to have access to health care,” says George K. Lewis, Ph.D., president and chief executive officer of ZetrOZ Systems, LLC. Instead of engaging in physical therapy multiple days a week, rural patients with joint pain may find it easier to take narcotics or receive injections every few months. Since 2013, NIMHD has funded studies led by Dr. Lewis to explore other options for pain management. Dr. Lewis’s research has focused on developing and testing wearable ultrasound devices for osteoarthritic pain, particularly in disadvantaged rural populations in upstate New York.
Osteoarthritis is a very common disease in which the cushioning around the joints wears down. It affects more than 30 million Americans, causing them chronic pain and making it hard for them to be active. People with this disease may manage their symptoms through weight loss, physical therapy, and medications to reduce pain and swelling.
Ultrasound, commonly used to image the inside of the body through soundwaves, can also be used at higher intensities to relieve pain,2 promote healing, and reduce swelling.3 Clinicians currently use ultrasound therapy for knee pain, treating it for several minutes a few times a week in the office. However, a review of clinical studies suggests that a few minutes may not be enough to provide long-lasting relief. Additionally, not everyone can get to the clinic or doctor's office several times a week, especially if they live in remote areas.
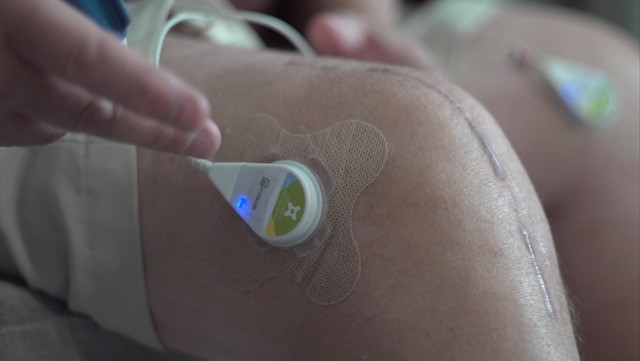
To address these problems, Dr. Lewis envisioned a wearable device that delivers low-intensity ultrasound waves over longer periods and can be used safely at home by anyone. By having a more constant low-intensity ultrasound dose, he predicted, patients could better control their pain. The creation stemmed from Dr. Lewis’ early interest in ultrasound technology. He grew up in a “medical-device ultrasound family”; his dad led ultrasound imaging research and product development for a medical technology company. Dr. Lewis worked in his father’s product development laboratory and carried on this interest through college.
Creating and Testing the Device
Dr. Lewis made his first version of a portable ultrasound device for home use back in 2008. “I invented it at my apartment in graduate school,” says Dr. Lewis. His wife needed ankle surgery, which carried a risk for complications due to limited blood flow in the feet. Dr. Lewis wanted to see if such a device could help her recover. The device he created appeared to help her heal and return to normal activities while managing her pain.
For his Ph.D. thesis in biomedical engineering, Dr. Lewis looked at using low-intensity ultrasound therapy for 20 to 60 minutes a session in order to help medications get across the blood-brain barrier to the brain. Recognizing that sustained ultrasound could help drugs reach other areas of the body, such as the knee and joint space, he started to explore new uses for sustained ultrasound treatment. Ultimately, this realization, along with existing data on low-intensity therapeutic ultrasound, led to his NIMHD-funded project.
Dr. Lewis and his team at ZetrOZ Systems (formerly ZetrOZ, Inc.) have seen great clinical success since their early studies in 2013. In the first phase, study participants wore the device for 4 hours a day for 8 weeks. They reported less pain, were more active, and took less pain medication than before they started using the device. Compared to people who wore a placebo device, study participants who wore an active device showed greater reductions in pain and more physical activity.
Getting the Device to Patients
Following these positive results, and additional studies on the device for treating tendon and myofascial pain, the U.S. Food and Drug Administration approved the ZetrOZ wearable ultrasound device in 2014, making it the first and only multi-hour ultrasound wearable device approved to increase circulation, reduce joint stiffening, and accelerate healing. Although physicians still manage the device and track patients’ use through a log and patient diary, patients use the device in their homes and do not need to see a clinician every week. Since the device is simple to operate, clinicians can teach people from any educational background how to use it. Furthermore, by having the option to use the device while at work, people may be able to return to their jobs much more quickly than they would using other pain therapies or none.
The device, called SAM® (Sustained Acoustic Medicine), has already helped tens of thousands of people. “It's game changing and engaging patients so that they’re actively involved in their care," says Dr. Lewis. He adds, “With improved range of motion, controlled pain, no drugs, and no injections, patients love the device, and it has been a commercial success.”
ZetrOZ Systems aims "to make SAM® devices affordable and acceptable so that they have a long-lasting impact and can help as many people as possible," says Dr. Lewis. Injured professional and college athletes are using the device, a nationwide network of SAM® device and insurance providers are in place for the treatment of work-related injuries, and the U.S. Department of Veterans Affairs has approved its use for veterans who have not experienced joint pain relief from other medical therapies. Twenty-six Veterans Affairs medical centers currently prescribe SAM®, and more than half of their patients are members of minority groups in rural communities.
Dr. Lewis says that more research is needed to understand the long-term benefits of using SAM® for osteoarthritis. Early studies suggest that it could help prevent joint replacements. Establishing a clinical trial to confirm this potential benefit will take some time, but Dr. Lewis and his team are hopeful as they continue to gain support.
References
- St. Sauver, J. L., Warner, D. O., Yawn, B. P., Jacobson, D. J., McGree, M. E., Pankratz, J. J., . . . Rocca, W. A. (2013). Why do patients visit their doctors? Assessing the most prevalent conditions in a defined U.S. population. Mayo Clinic Proceedings, 88(1), 56–67. doi:10.1016/j.mayocp.2012.08.020
- Muftic, M., & Miladinovic, K. (2013). Therapeutic ultrasound and pain in degenerative diseases of musculoskeletal system. Acta Informatica Medica, 21(3), 170–172. doi:10.5455/aim.2013.21.170-172
- Miller, D., Smith, N., Bailey, M., Czarnota, G., Hynynen, K., Makin, I., & American Institute of Ultrasound in Medicine Bioeffects Committee. (2012). Overview of therapeutic ultrasound applications and safety considerations. Journal of Ultrasound in Medicine: Official Journal of the American Institute of Ultrasound in Medicine, 31(4), 623–634. doi:10.7863/jum.2012.31.4.623
Posted October 4, 2018